Chemical Elements and Electrical Charges
What is a Chemical Element?
A chemical element is a pure substance made of only one type of atom.
Each atom of an element has the same number of protons in its nucleus — this number is called the atomic number (Z).
For example:
• Hydrogen (H) has 1 proton → atomic number = 1
• Helium (He) has 2 protons → atomic number = 2
• Oxygen (O) has 8 protons → atomic number = 8
There are over 118 known elements in the periodic table, each with unique properties.
Each atom of an element has the same number of protons in its nucleus — this number is called the atomic number (Z).
For example:
• Hydrogen (H) has 1 proton → atomic number = 1
• Helium (He) has 2 protons → atomic number = 2
• Oxygen (O) has 8 protons → atomic number = 8
There are over 118 known elements in the periodic table, each with unique properties.
Not mentioning isotopes that have been identified.
Structure of an Atom
An atom has three main particles:1. Protons → positive charge (+1)
2. Electrons → negative charge (–1)
3. Neutrons → no charge (0)
They are arranged like this:
• Nucleus: contains protons and neutrons
• Electron cloud: electrons orbit around the nucleus
Electrical Charges in Atoms
In a neutral atom, the number of protons = electrons, so the charges cancel out.Example:
Hydrogen atom → 1 proton (+1) and 1 electron (–1) → total charge = 0
Ions — When Atoms Gain or Lose Electrons
Atoms can gain or lose electrons to become ions, which carry electrical charge:• Cation (+): Atom loses electrons → more protons than electrons
Example: Na → Na⁺ (sodium ion)
• Anion (–): Atom gains electrons → more electrons than protons
Example: Cl → Cl⁻ (chloride ion)
Isotopes — Same Element, Different Neutrons
Atoms of the same element can have different numbers of neutrons, forming isotopes.Example: Hydrogen has three isotopes:
• Protium (¹H): 1 proton, 0 neutrons
• Deuterium (²H): 1 proton, 1 neutron
• Tritium (³H): 1 proton, 2 neutrons
All have the same electrical charge (neutral when un-ionized), but different atomic masses.
Table to help you identify Charges of Elements
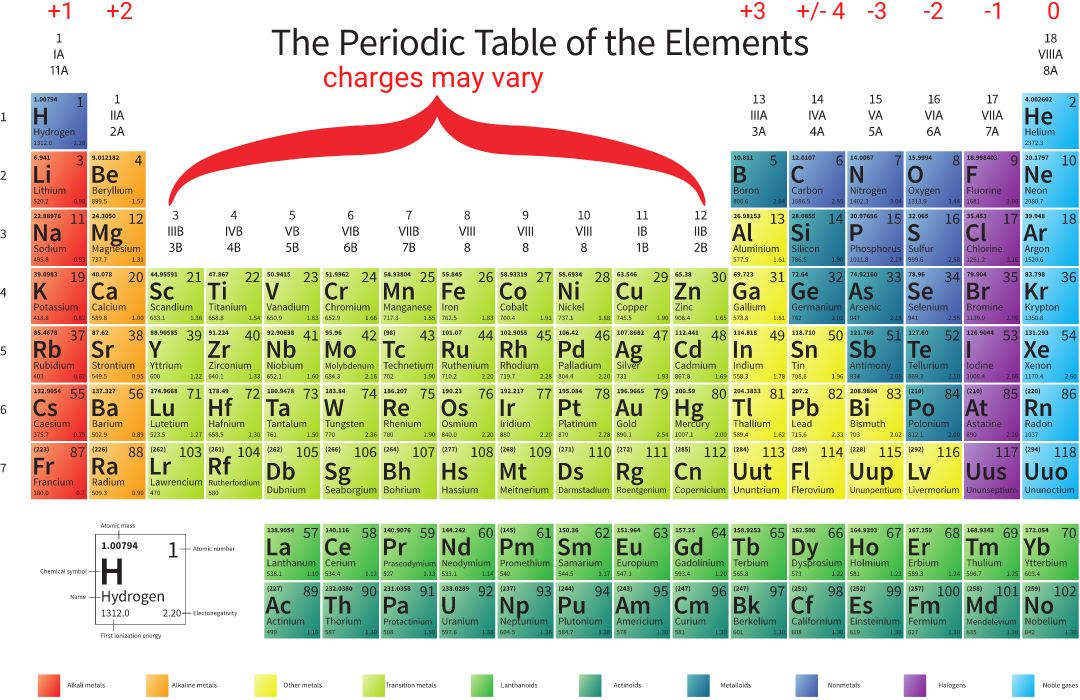
Starting with Group I, elements have a positive charge. Look at the last digit in the number to determine the charge. Group 1 has a +1 charge. Group 2, +2. Skip the Transition Metals (Groups 3 – 12) because elements in that region may have a variety of charges. Group 13 has a +3 charge, and Group 14 may have a + or – 4 charge. Starting with Group 15, the charges are negative, starting with a -3 charge and decreasing to 0 by Group 18.
This information becomes important when you want to determine how elements combine with one another to form ionic bonds.
