Periodic Table of Chemical Elements
Basic Chemistry: The Periodic table of elements is a tabular arrangement of all known chemical elements, organized on the basis of their atomic numbers and internal configuration*, in the order of increasing electron number. Atomic number is a number of protons in the nucleus, electron configuration is the distribution (position) of electrons of an atom or molecule.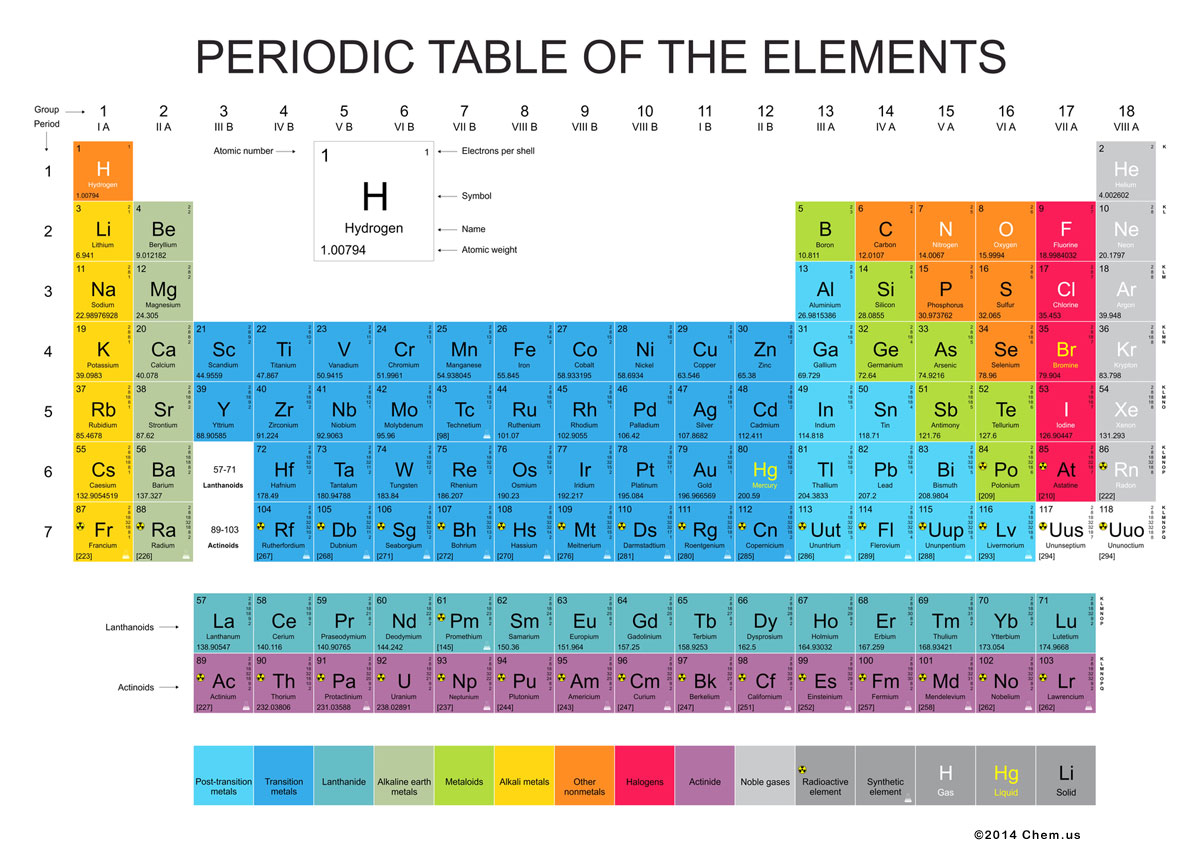
Click picture to get full view
Internal Configuration
Recurring chemical properties: Elements in periodic table are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 rows, with a double row of elements below that did not fit standard table format - Lanthanoids and Actinoids.This table format is generally attributed to a russian chemist Dmitri Mendeleev who in 1869 published the first widely recognized periodic table. He developed it to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery of newer elements and the development of new theoretical models to explain chemical behavior.
Proof of Existence of Chemical Elements
All elements from atomic numbers 1 (Hydrogen) to 118 (Ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.
Element Groups
Chemical elements are divided into 10 groups. Each group has it's own special properties. See corresponding colours on the table.
- Post-transition metals
- Transition metals
- Lanthanide
- Alkaline earth metals
- Metaloids
- Alkali metals
- Other non-metals
- Halogens
- Actinide
- Noble gases
- Radioactive elements, and
- Synthetic elements
